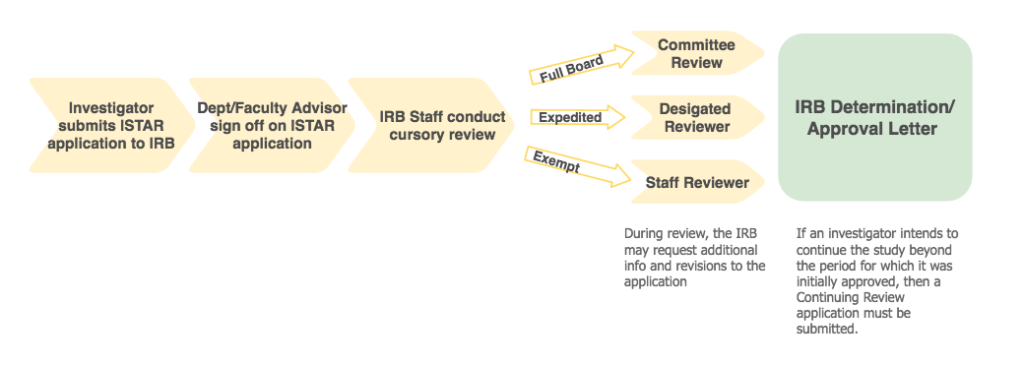
How to get IRB review
- Complete the Human Subjects Research TrainingThe Human Subjects Research course is required of all USC investigators and personnel on the IRB application.
The IRB reviewer will notify the applicant if there are additional requirements needed for IRB approval (such as Good Clinical Practice training for clinical trials) . - Submit an ISTAR ApplicationApplications for IRB review must be submitted online through the iStar system. All personnel listed in the IRB application must establish an iStar account by logging into the site with their USC ID or following the steps for users without a USC ID.
IRB Approval Process
- Investigator Submits Study via iStar: Investigators must indicate if the application requires exempt, expedited, or full board review. (The IRB staff will correct the selected level of review, if necessary.)
- Faculty Advisor/Department Sign-off: Once the application is submitted, the faculty advisor or department chair signs-off on the application. This sign-off represents review of scientific merit, and availability of resources at the department level.
- IRB Office: After department/faculty advisor approval is obtained, a cursory review is conducted by the IRB staff. IRB staff evaluates the protocol and supporting documents (e.g., consent form, recruitment materials). If a study is approved as exempt or determined to be “not human subjects research,” a determination letter is promptly issued by IRB staff.
Studies designated as expedited or full board are reviewed by a designated reviewer or the full board, respectively. - Study Approved and PI Notified: The researcher will be notified through an iStar generated email when the study has been approved.
- Significant changes to a study after approval must be submitted and reviewed by the IRB prior to implementation.
How long does IRB review take?
IRB applications should be submitted at least 1 month prior to the intended research start date.
All IRB submissions are reviewed in the order received (new studies, amendments, continuing reviews, and responses to contingencies). Studies will not be “pulled out of queue” however, the IRB may be able to accommodate funding related deadlines with advance notice.
Full Board studies are reviewed per the IRB meeting deadlines:
After Approval: When to contact the IRB
After IRB approval of a study, there are occasions when an additional IRB submission is required to either modify the project, continue the project beyond its initial term of approval, or to report an unexpected adverse event that occurred during the research.
Continuing Review (Renew Approval)
To continue the study beyond the period for which it was initially approved, a Continuing Review application must be submitted. Studies that require Continuing Review will receive email notifications from iStar with instructions for how to submit a Continuing Review application.
Amendments
Investigators must seek IRB approval before making any changes to an approved research study. Changes to the study protocol may not be implemented until approved by the IRB. When the IRB approves the amendment, the modified study replaces the previously approved study.
However, when a change is necessary to eliminate immediate hazard to the subject a change may be implemented without IRB approval and the IRB must then be notified right away.
See Reportable Events
Reportable Events
During the course of research situations may occur that must be reported to the IRB. These include complaints from subjects, protocol deviations, adverse events, and significant new information or findings. An application for submitting a reportable event can be accessed in iStar. Detailed information about reportable events can be read in the policies of the Human Subject Protection Program.
Reportable events include:
- Complaints- dissatisfaction, a breach of subjects rights/research ethics expressed by a subject or subject’s representative
- Protocol Deviation or Error- when research procedures are intentionally or accidentally not followed.
- If an apparent, immediate hazard to participants is identified, participants must be notified and corrective actions implemented as soon as possible. The IRB must be informed about these occurrences and the investigator must promptly submit a reportable event to the IRB.
- Adverse events- any untoward or unfavorable medical occurrence in a human subject, including any abnormal sign (for example, abnormal physical exam or laboratory finding), symptom, or disease, temporally associated with the subject’s participation in the research, whether or not considered related to the subject’s participation in the research.